7.6 Accuracy
Accuracy is defined as the closeness of agreement between a measured quantity value and the true quantity value of the measurand. The concept "measurement accuracy" is not a quantity and is not given a numerical quantity value. A measurement is said to be more accurate when it offers a smaller measurement error. As measurement error is understood as the difference between the measured quantity and its reference quantity value, accuracy is affected by both trueness and precision of the measurement. An illustration of the concept of accuracy, versus those of precision and trueness is given below:
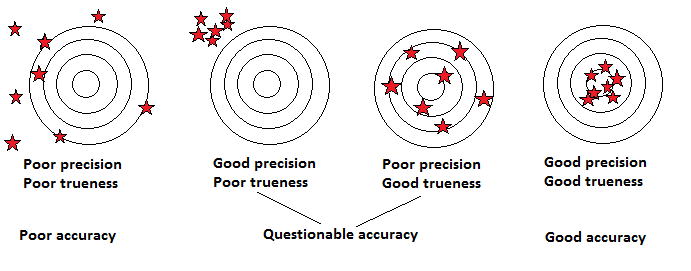
One approach based on combined assessment of the a priori acceptance criteria defined for trueness and precision of the results and the assignment of the status "acceptable" or "not acceptable" accordingly has been adopted by the proficiency test schemes of the IAEA environmental laboratories since 2006 (Shakhashiro et. al., 2006). If the a priori established maximum acceptable percentage bias and precision for the measurement results are MAB and MAP respectively, then a result ymeas is considered as acceptable if its bias B and precision P comply with the following two conditions:
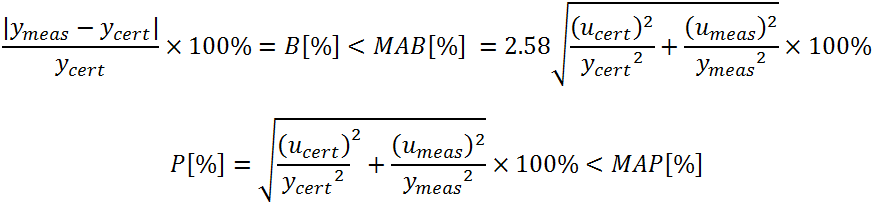
where ycert and ymeas are reference and measurement result values and; ucert and umeas are the uncertainties of the reference value and that reported for the measurement result, respectively. In cases when none of the criteria is fulfilled, the measurement result is obviously evaluated as non-acceptable.
If one of the two criteria is fulfilled and the other is not, a further check has to be performed. If the observed bias is larger than the MAB, then the result is considered as non-acceptable. If the bias is smaller than the MAB, then a warning is flagged, and further investigation must be conducted as to determine the factors influencing the observed poor precision.
Reference: A. Shakhashiro, A. Fajgelj and U. Sansone, Comparison of Different Approaches to Evaluate Proficiency Test Data, in Combining and Reporting Analytical Results, Eds. Maria Belli, A Fajgelj, Umberto Sansone, Royal Society of Chemistry, 2006, pp. 220-228, ISBN: 978-0-85404-848-9.