2. X-rays physics
The constituent particles forming an atom are protons, neutrons and electrons. Protons and neutrons are known as nucleons and form the nucleus of the atom.
Atomic number Z represents the number of protons and number of electrons in an atom.
Atomic mass number A is the number of nucleons in an atom (i.e. number of protons Z plus number of neutrons N in an atom: A = Z + N).
There is no basic relation between A and Z, but the empirical relationship furnishes a good approximation for stable nuclei.
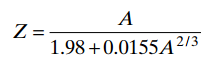
The origin and properties of the x-rays are closely related to interactions in the atom electron cloud.
