5.1.1 XRF spectrometry
Among the emission techniques, X-ray Fluorescence spectrometry is the most widely spread technique for elemental analysis. Characteristic radiation is excited with photons (either gamma or x-rays) and that is the reason for the technique to be specifically called fluorescence.
X-ray generators are the most commonly used source, because the spectral distribution of the x-ray tubes emission can be tuned for specific measurements by making a proper selection of the excitation high voltage and by using different types of modifiers, such as filter, secondary targets and scattering devices. Radioactive sources can also be used for excitation without the need for a power supply, and allowing their easier use in small portable instruments. Synchrotrons provide polarized and tuneable monochromatic radiation for excitation and the x-ray beams can be very small and very intense, thus featuring improved spatial resolution and detection limits.
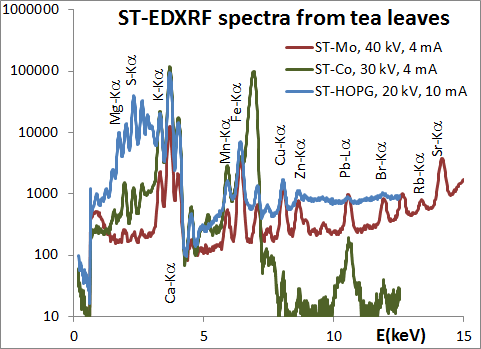
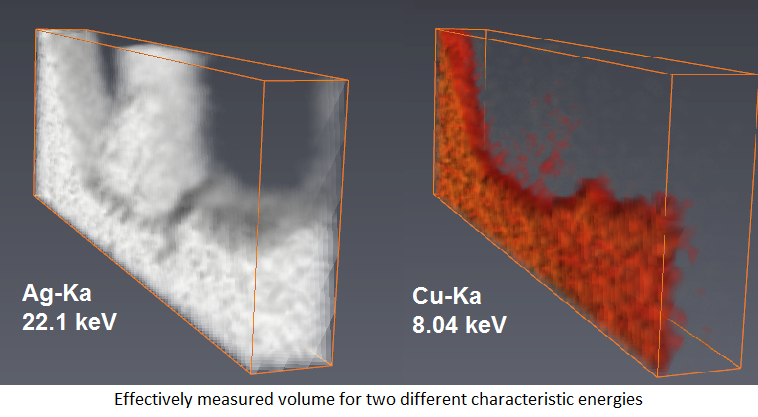
As the attenuation of x-rays depends on their energy, the characteristic radiation from different elements emerges from different thickness layers under the sample surface. Therefore, for the analysis of bulk samples a good homogenization of the sample is required.
Depending on the principle used to sort the detected radiation, two main configurations of XRF spectrometers exist: Wavelength and energy dispersive XRF spectrometers (WDXRF and EDXRF, respectively). About 80 % of the instruments are of WDXRF type, and are widely spread in laboratories dealing with specific analysis, such as cement, steel and mineral processing industries. EDXRF instruments are more popular in laboratories facing variable analytical challenges. The large variety of instrument designs and selection of components make the XRF technique a quite versatile tool for elemental analysis of almost every type of samples and analytical problems.