2.6.1 Photoelectric absorption
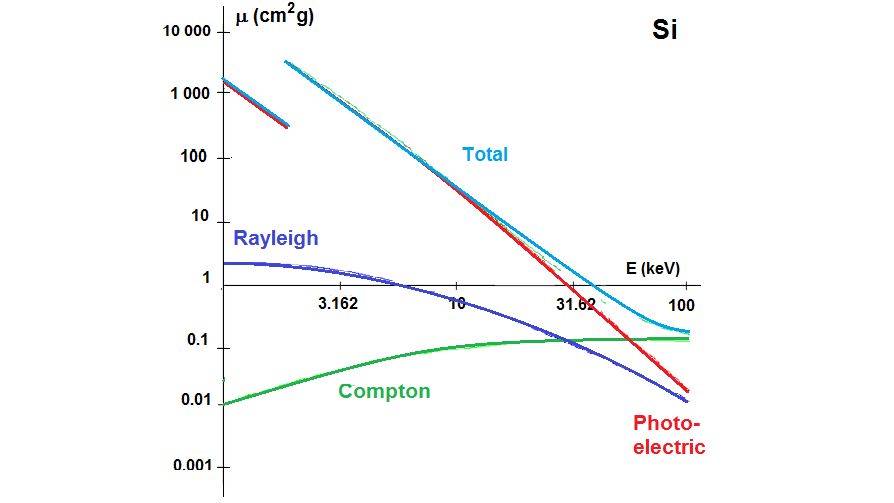
Photoelectric absorption is the more probable interaction and is caused by the absorption of the x-ray energy by the atom, leading to an ionization process, as depicted in animation in section 2.4.2. Photoelectric absorption does not depend on the angle of incidence of the radiation, and increases with the atomic number of the attenuating material.
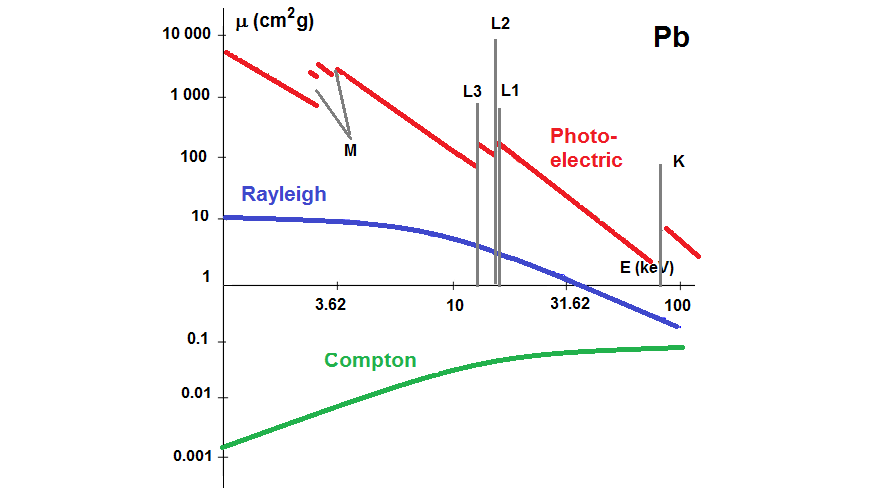
The lower the energy of the x-rays, the higher their absorption in the material. For photons with higher energy the absorption decreases. If the x-ray energy exceeds that required for expelling one electron from the M, L or K shells, the probability of absorption increases and discontinuities in the form of jumps originate in the cross section dependence, as depicted in the photoelectric absorption cross section for Si and Pb. Measuring sample absorption at energies above and below the absorption edge provide information on the content of a given element and is used in different absorption techniques.
When the apparently sharp x-ray absorption discontinuities are examined at high resolution they are found to contain a fine structure that extends in some cases to a few hundreds of electron volts above the energy of the absorption edge. Such structures in the region up to 50 eV above the edge energy are conditioned by transitions of the ejected electrons to unfilled discrete energy states of the atom or the molecule rather than to the continuum of states beyond a characteristic energy and are referred to as X-ray Absorption Near Edge Structure (XANES). Another structure is overlapping and extending to the region to about 200 eV above the absorption edge (EXAFS, Extended X-ray Absorption Fine Structure) due to interference effects on the Broigle waves of the ejected electrons by the molecular or crystalline spatial ordering coefficients of the neighbouring atoms. Such structures provide information for speciation or molecular identification.