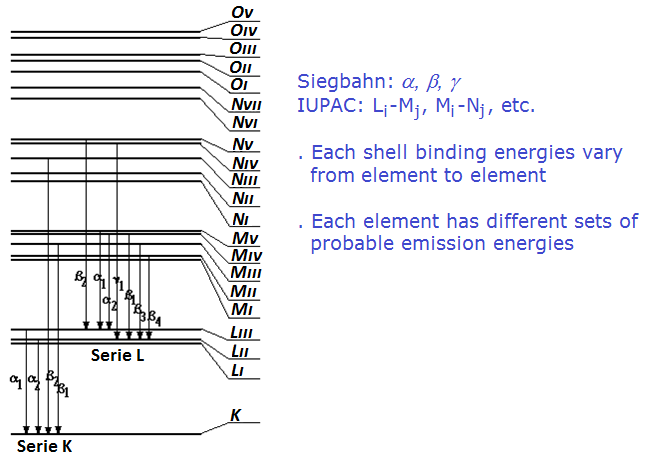
2.5 Characteristic radiation
Historically the x-ray emission groups of lines were denoted based on the shells the electron would occupy after a transition. If the electron moves to a K shell vacancy, the emission is called K. If the transition leads to occupying a vacancy in the L shells then is call L, and so on. Such denotation was established by Siegbahn and the characteristic emission energies within one group (K, L or M) were denoted based on the probability to occur (α for the more intense lines, β for the next, γ and so on).
The notation according to the IUPAC is based on the end and origin shells for each transition, as represented in the Figure below. Therefore Kα1 emission corresponds to K-L3, Kβ1 to K-M3, Lα1 to L3-M5, Lα2 to L3-M4 and so on.