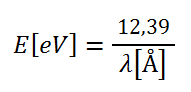2.2 Energy and wavelength
The relationship between Energy and wavelength is given by the Planck-relation:
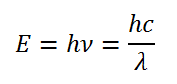
where:
E = Energy (J)
h = Planck constant (6.62606896×10-34 Js)
v = frequency (Hz, s-1)
c = speed of light in vacuum (299792458 ms-1)
λ = wavelength in meters (s-1)
X-ray energy and wavelength are conventionally expressed in electron volts (eV) and Amstrongs (Å), respectively. One electron volt is the amount of energy gained (or lost) by the charge of a single electron moved across an electric potential difference of one volt (1 eV = 1.602176565(35) x 10-19 J), 1 Å = 10-10 m. Therefore, the Planck relation can be rewritten as: