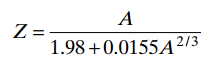2.1 Atomic and nuclear structure
The constituent particles forming an atom are protons, neutrons and electrons. Protons and neutrons are known as nucleons and form the nucleus of the atom.
- Atomic number Z represents the number of protons and number of electrons in an atom.
- Atomic mass number A is the number of nucleons in an atom (i.e. number of protons Z plus number of neutrons N in an atom: A = Z + N).
- There is no basic relation between A and Z, but the empirical relationship furnishes a good approximation for stable nuclei.